[This article was co-written by Krystle Salvati of the Macaulay Honors College at John Jay College (CUNY) based on a paper she wrote in one of my courses.]
At the very ends of our chromosomes, there are structures called telomeres. Telomeres are made of a specific DNA sequence – TTAGGG – repeated thousands of times. As we begin our lives, the chromosomes in most of the cells in our body have telomeres with about 2,500 copies of the repeat sequence. This highly repetitive DNA serves as a “cap” on the tips of our chromosomes.

Because of the constraints of how DNA is copied, telomeres get a tiny bit shorter each time a chromosome is copied in preparation for cell division. Exceptions are the telomeres in certain skin cells and those of our GI tract, as well as hematopoietic stem cells (blood cell progenitors), gamete precursors (sperm and egg), and cells that have been “immortalized” such as cancer cells and most laboratory-cultured cell lines. Because those cells are meant to divide forever, they have ways to maintain their telomeres forever.
For most other cells, the gradual shortening of the telomeres places an upper limit on the number of possible cell divisions. As telomeres shorten to a critical length, cells undergo a process called replicative senescence, where they stop dividing and mostly cease to function. As such, telomeres are aggressively studied in the fields of cancer biology and aging.
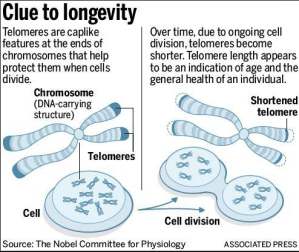
The telomeres of white blood cells (leukocytes) are also commonly studied as markers of physiological stress. Although telomeres always shorten with age, they prematurely shorten in a certain kind of cell when that cell is “busier” than usual. This is because each round of cell division eats away at the chromosome ends. Leukocytes are the cells of the immune system, so the leukocytes of someone who is affected by chronic stress, infection, or injury have shorter than expected telomeres due to the overactivity of leukocytes associated with the stress response.
With a simple blood draw, researchers can isolate circulating leukocytes and then measure the length of their telomeres. The leukocyte telomere length (LTL) corresponds roughly to age, augmented by the degree of physiological stress that the person has undergone. Those with chronic illnesses, especially autoimmune diseases, frequent infections, and other inflammatory conditions, display telomeres that are shorter than their age would predict. As such, LTL is often used as a rough marker of general health.

Recently, Flannagan and colleagues published a fascinating article in the American Journal of Human Biology examining leukocyte telomere length (LTLs) in several Mesoamerican populations and attempting to correlate them to various measures of health and chronic stress. Their study focused on families living in six Mesoamerican countries: Belize, Costa Rica, Honduras, Mexico, Nicaragua, and Panama. At least two dozen families were examined in each country with researchers drawing blood to obtain the LTL measurements as well as taking other biometrics such as height and weight, for calculation of Body Mass Index (BMI), as well as age, education, family size, and other social factors.
Unsurprisingly, LTL correlated well with age in all Mesoamerican sub-populations and shortened telomeres were also observed in those with a high BMI and those reporting chronic illnesses. Across all six countries, mothers’ telomere length correlated well with that of their children (corrected for age), unsurprising given that telomeres are chromosomally inherited. For men, telomere length was shorter if they did not have “food security.”
In addition, the researchers found something quite unexpected: LTL correlated surprisingly well with nationality. In fact, nationality was an even better predictor of LTL than family relationships, when correcting for age and chronic illness. This correlation between nationality and LTL was observed in both adults and children. Flannagan and colleagues speculate that the aforementioned correlations could be caused by “lifestyle characteristics or ecological exposures.” For example, LTL could be affected by “stress associated with political and social unrest, national health and social welfare policies, or by differences in standards of living between countries.”

Another variable that may connect nationality and telomere length is healthcare access itself. In Flannagan, we see that the countries in Mesoamerica with the longest average telomeres among women are, in order, Mexico, Panama, and Costa Rica. Of the six countries examined, these have the greatest access to healthcare among the general population. Indeed, the healthcare systems in these countries are among the best in Latin America at providing universal access to their citizens. In fact, these three are all in the top five of International Living’s ranking of the world’s best healthcare systems and in all three cases, the ubiquity of healthcare access is specifically credited. In contrast, the shortest telomeres, by far, were observed in Belize and Honduras, two countries that struggle to provide access to basic healthcare for many of their citizens.
It is tempting to interpret this correlation as merely coincidental with economic development. Indeed, other studies have found that shorter telomeres correlate with poverty (here and here) and per-country wealth inequality (here and here). Researchers in Scotland have found that telomere length is associated specifically with household income. However, two countries in the Flannagan data set, Belize and Nicaragua, buck this trend. Belize is wealthier than most countries in Mesoamerica, but with lower access to healthcare, while Nicaragua strives to provide universal health care despite being one of the poorest countries in the region. These exceptions prove the rule: it appears that, healthcare access per se, not economic development, is the factor that best correlates with telomere lengths among Mesoamerican countries.
Furthermore, it has recently been revealed that Costa Rica has considerably lower mortality and a longer life expectancy than the United States, despite the latter having seven times higher per capita personal income and spending many times more on healthcare. Although Rosero-Bixby and Dow have linked this health disparity to far greater wealth inequality in the United States, a simpler though not unrelated factor, and not mutually exclusive, may be that Costa Rica has universal healthcare coverage and the United States does not.
That the correlation between nationality and LTL observed by Flannagan is independent of other health indicators argues that it is not just healthcare treatments that are beneficial to health, but rather, healthcare access itself is beneficial to general health. The chronic psychological stress of healthcare insecurity may lead to chronic physiological stress and illness. Indeed, connections between telomere length and both psychiatric illness and life stress have already been found.
Our telomeres are telling us that lack of healthcare access is a considerable health burden in and of itself.
-Krystle Salvati and Nathan H. Lents
Fascinating. The pivotal role that healthcare access plays in telomere length and life expectancy is striking. Very much the kind of strong scientific evidence that some political groups believe should be ignored.
I was curious, though, for a little more explanation about why and how skin and other cells are “meant to divide forever” while cell division “eats away” at the telomeres of other cells. The contrast seems dramatic and important.
Thanks for the great post.
Brock
LikeLike
There are a few cell types in the body that are endlessly regenerating – skin cells and the cells that make sperm are good examples. Obviously, if their telomeres shortened every round of division, they would run out of telomeres very early in life. In these cells, a gene is turned on for an enzyme called telomerase. Telomerase extends the telomeres every round of division so that they don’t shorten. But most cells don’t have the telomerase gene turned on, so their telomeres gradually shorten throughout life. Basically all cancer cells find a way to turn the telomerase gene on to make themselves immortal. It’s one of the key steps in the genesis of cancer. Does that help clarify?
LikeLike
Very much so. Thanks.
LikeLike
I would like to pose a question. Would it be possible to research the notion that it is not humans that came from apes, but rather it is that apes came from humans?
In my religion, you may scoff at that, we believe that there were certain people who were turned into monkeys.
I think it would be important to see if there is a link that way.
LikeLike